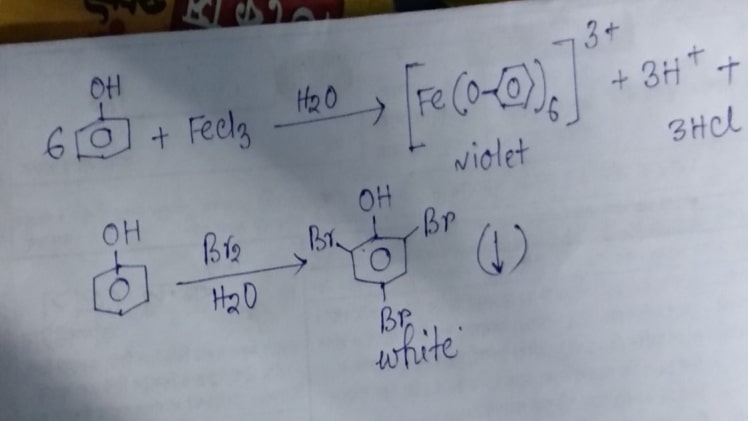
How will you distinguish between alcohol and phenol?
To study any topic related to organic, inorganic, or physical chemistry it is important to first finish the prescribed textbook of your respective state boards. In case of CBSE board the prescribed textbook is NCERT. The language used in this book is relatively simpler as compared to any other reference book or any other state board book. Therefore, it is important to finish the book first to have a sound knowledge or grip over the subject chemistry. Once the candidate is through with the textbook it is advisable to switch to any other reference book of the student’s choice.
This topic is found in NCERT book chapter 11. Some basic concepts regarding alcohol and phenol are mentioned, IUPAC nomenclature, discuss the reactions involved in the preparation of alcohol, preparation of ethers, correlation physical properties of ethers, alcohols and phenols with their structures and chemical reactions of classes of compounds namely alcohol, phenol, ether.
Now, how can the term alcohol easily be defined? Alcohol is one of the most typically determined compounds found in nature. They incorporate as a minimum one hydroxyl family group(-OH) connected to a carbon atom of a hydrocarbon or an alkyl group of compounds. Further, the sort of carbon connected to the hydroxyl family determines whether or not the alcohol is a primary, secondary, or tertiary compound. the structure of atom diagram can be studied from the textbook.
Now discussing some of the common physical properties of the alcohol. It can be categorized into three parts namely the boiling point, solubility, and acidity. Since there is presence of hydrogen bond between the hydroxyl groups the boiling point is maintained at slightly higher levels. As far as solubility of the alcohols are considered, the condition states that if the hydrogen bonds are hydrophilic and form hydrogen bonds with water then alcohols are soluble in water. Lastly, alkoxides are formed alcohol reacts with sodium, potassium, and other active metals. The nature of this reaction is acidic.
Next topic is phenol. So, what is phenol in simpler terms? Phenols or phenolics are chemical substances that include a hydroxyl family member connected to an aromatic hydrocarbon. They are called a subset of alcohol however show off exceptional physical and chemical characteristics in comparison. They also are referred to as carbolic acids and are used to put together nylons, herbs, detergents, and different pharmaceutical products. Structure of the same can be studied from the textbook which is freely available on the NCERT website.
The physical property of phenol is fairly simple, and it can be categorized into two parts namely boiling point and solubility. Phenols unlike most hydrocarbons have higher boiling point. As far as solubility is concerned it is highly soluble in water.
There are many differences between alcohol and phenol. Some of them are discussed below.
Alcohol (organic compound) that contains one or more hydroxyl functional groups connected to a saturated carbon atom whereas Phenols are organic compounds that consist of a hydroxyl group attached to a group of hydrocarbons or arene. Alcohol and Phenol consists of aliphatic hydrocarbons and aromatic hydrocarbons respectively. Alcohols are less acidic whereas Phenols are more acidic in comparison with alcohol and need to be diluted before use. Alcohol is used as an ingredient in alcoholic beverages, pharmaceuticals, ink, and various other chemical products whereas Phenols are commonly used as antiseptic agents. Under standard temperature and pressure Alcohols are colorless and are liquid in nature whereas Phenols are crystalline but are also colorless in nature. Under litmus paper test Alcohols being neutral in nature show no reaction on litmus paper or other tests whereas Phenols being acidic in nature change the color of litmus paper to red. While Alcohols stay indifferent or rather do not react with sodium hydroxide (NaOH), phenoxides are formed when Phenols react with sodium hydroxide (NaOH). Coupling reaction – azo dye test Phenol give orange color; alcohol do not give any reaction. Bromine water test, When Phenol is added to Br2water they give white ppt. Due to formation of two three and five tri nitro phenol but alcohol do not give any such test.
Do you know the answer of the below question?
- The intermediate species involved in the acid catalyzed dehydration of alcohol is?
- Free radical
- Carbocation
- Carbanion
- Carbene
In class 11th NCERT book the topic of alcohols and phenol is mentioned in scattered form throughout the book in various chapters whereas in grade 12th there is a dedicated chapter for this topic only where every aspect of this topic is explained in detail. The entire topic is summarized below.
the different methods for the preparation of alcohol: Alcohols are prepared through the hydrolysis of halides, hydration of alkanes, and hydroboration of alkenes. the different compounds used for the preparation of phenol: Phenols are prepared using haloarenes, benzene sulphonic acids, cumene, and diazonium salts. Yes, both alcohols and phenols are polar. Yes, both phenols and alcohols can undergo oxidization. In 1834 Phenol was discovered by a German chemist Dr. Friedlieb Ferdinand Runge.